Fortunately, the way rocks dissolve is kind of important, so at least I've got that going for me. Look at the formulas to the left (1a-3a): Step 1a forms a weak acid from water and carbon dioxide.
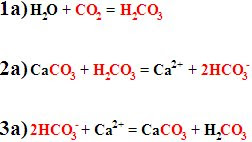 Step 2a uses that acid to dissolve a carbonate rock (limestone). This happens whenever rain mixed with CO2 falls on these rocks, and the rain eventually washes the weathering products into the ocean. Step 3a is what occurs when organisms living in the ocean use these weathering products to make their shells out of limestone. It's the exact reverse of step 2a. Now look at the carbon atoms in the equations (they're all highlighted in red): One carbon atom comes from the air, and one comes from the original limestone. In the end there is one carbon atom in the new limestone, and one has turned back into the weak acid (which is effectively the same as being returned to the air). So this process doesn't change the amount of CO2 in the air at all.
Step 2a uses that acid to dissolve a carbonate rock (limestone). This happens whenever rain mixed with CO2 falls on these rocks, and the rain eventually washes the weathering products into the ocean. Step 3a is what occurs when organisms living in the ocean use these weathering products to make their shells out of limestone. It's the exact reverse of step 2a. Now look at the carbon atoms in the equations (they're all highlighted in red): One carbon atom comes from the air, and one comes from the original limestone. In the end there is one carbon atom in the new limestone, and one has turned back into the weak acid (which is effectively the same as being returned to the air). So this process doesn't change the amount of CO2 in the air at all.Now look at this second set of formulas. Step 1b is the same as step 1a above, except that the amounts of products and reactants are doubled, and a weak acid is created by combining carbon dioxide with rainwater. In Step 2b, the two acid molecules dissolve a single silicate rock, producing the same weathering products as above, plus an extra water molecule and a molecule of quartz (we can ignore these last two from here on). Step 3b is again the same as step 3a above, as sea-critters make their shells out of limestone - but now look at the carbon molecules in this equation: Both carbon molecules come from the air, and although one is returned to the air at the end (okay, to the acid, but remember that they're effectively the same, as far as we're concerned), the other is now stored in rock!
So, yes, I did just use an exclamation point in a story about how rocks dissolve, but the fact that I'm a giant nerd isn't the take-home message here. The message is that the weathering of silicate minerals takes CO2 from the air and ends up storing it in rock! (another one, ha! (!)) So if you've ever wondered to yourself what controls the amount of CO2 in the atmosphere (essentially what causes climate change), this is it.
Which brings us to my job: I measure the chemistry of river water, which is essentially the sum of the weathering products from everywhere upstream conveniently mixed into a single bottle, and try my darndest to figure out which of these reactions they come from, and how much CO2 is being consumed by these processes.

1 comment:
Well that is an excellent summary of chemical weathering. But still, the only thing I took home from it is that you are a giant nerd. I mean we all are, but you especially :)
Post a Comment